Project
Organ-on-a-chip models for safety testing of regenerative medicine products
| Primary Investigator: | Prof Hazel Screen Queen Mary University of London |
| Co-investigators: | Prof Martin Knight Queen Mary University of London |
| Prof Alicia El Haj University of Birmingham | |
| Funder: | MRC Medical Research Council |
| Project dates: | 24-08-2020 to 23-08-2023 |
| Centre dates: | 24-08-2020 to 23-08-2023 |
Regenerative medicine aims to replace or regenerate human cells, tissues or organs to restore or establish normal function. Any newly developed products much be subject to stringent pre-clinical safety tests before use in humans. Currently, we mainly use animal models for pre-clinical safety testing, but not only does this bring ethical concerns, but animals are biologically different to humans, so sometimes the testing can provide poor indication of the product is actually safe in humans. We urgently need more representative models which contain human cells, to see how our bodies will react to the new products.
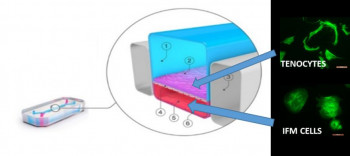
Our project is focused on the design of new, improved models for the predictive safety testing of new regenerative medicine products. The models we will create are called "organ-on-a-chip" models, as they create a miniature biologically correct human organ on a small device reminiscent of a computer chip. We can input the new regenerative medicine products into the organ of choice built onto the chip, to see how that organ will respond.
We will work one of the largest manufacturers of organ-on-a chip models, a company called Emulate Inc. They have already created organ-on-a chip models of lung and liver which we will establish in our laboratories, before we create brand new models of the different musculoskeletal tissues prone to injury, so we can test new regenerative medicine products developed for any of these tissues.
Working with Emulate is important as they have developed a commercially available platform within which we can develop the models, making it easier and faster to translate our new models to commercial products, available to all companies, clinicians and researchers trying to create new drugs or regenerative medicine products.